Biology:Iron in biology
Iron is an important biological element.[1][2][3] It is used in both the ubiquitous iron-sulfur proteins[1] and in vertebrates it is used in hemoglobin which is essential for blood and oxygen transport.[4]
Overview
Iron is required for life.[1][2][3] The iron–sulfur clusters are pervasive and include nitrogenase, the enzymes responsible for biological nitrogen fixation. Iron-containing proteins participate in transport, storage and use of oxygen.[1] Iron proteins are involved in electron transfer.[5] The ubiquity of Iron in life has led to the Iron–sulfur world hypothesis that iron was a central component of the environment of early life.[6][7][8][9][10]
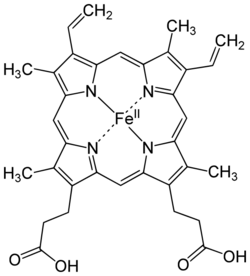
Examples of iron-containing proteins in higher organisms include hemoglobin, cytochrome (see high-valent iron), and catalase.[1][11] The average adult human contains about 0.005% body weight of iron, or about four grams, of which three quarters is in hemoglobin – a level that remains constant despite only about one milligram of iron being absorbed each day,[5] because the human body recycles its hemoglobin for the iron content.[12]
Microbial growth may be assisted by oxidation of iron(II) or by reduction of iron (III).[13]
Biochemistry
Iron acquisition poses a problem for aerobic organisms because ferric iron is poorly soluble near neutral pH. Thus, these organisms have developed means to absorb iron as complexes, sometimes taking up ferrous iron before oxidising it back to ferric iron.[1] In particular, bacteria have evolved very high-affinity sequestering agents called siderophores.[14][15][16]
After uptake in human cells, iron storage is precisely regulated.[1][17] A major component of this regulation is the protein transferrin, which binds iron ions absorbed from the duodenum and carries it in the blood to cells.[1][18] Transferrin contains Fe3+ in the middle of a distorted octahedron, bonded to one nitrogen, three oxygens and a chelating carbonate anion that traps the Fe3+ ion: it has such a high stability constant that it is very effective at taking up Fe3+ ions even from the most stable complexes. At the bone marrow, transferrin is reduced from Fe3+ and Fe2+ and stored as ferritin to be incorporated into hemoglobin.[5]
The most commonly known and studied bioinorganic iron compounds (biological iron molecules) are the heme proteins: examples are hemoglobin, myoglobin, and cytochrome P450.[1] These compounds participate in transporting gases, building enzymes, and transferring electrons.[5] Metalloproteins are a group of proteins with metal ion cofactors. Some examples of iron metalloproteins are ferritin and rubredoxin.[5] Many enzymes vital to life contain iron, such as catalase,[19] lipoxygenases,[20] and IRE-BP.[21]
Hemoglobin is an oxygen carrier that occurs in red blood cells and contributes their color, transporting oxygen in the arteries from the lungs to the muscles where it is transferred to myoglobin, which stores it until it is needed for the metabolic oxidation of glucose, generating energy.[1] Here the hemoglobin binds to carbon dioxide, produced when glucose is oxidized, which is transported through the veins by hemoglobin (predominantly as bicarbonate anions) back to the lungs where it is exhaled.[5] In hemoglobin, the iron is in one of four heme groups and has six possible coordination sites; four are occupied by nitrogen atoms in a porphyrin ring, the fifth by an imidazole nitrogen in a histidine residue of one of the protein chains attached to the heme group, and the sixth is reserved for the oxygen molecule it can reversibly bind to.[5] When hemoglobin is not attached to oxygen (and is then called deoxyhemoglobin), the Fe2+ ion at the center of the heme group (in the hydrophobic protein interior) is in a high-spin configuration. It is thus too large to fit inside the porphyrin ring, which bends instead into a dome with the Fe2+ ion about 55 picometers above it. In this configuration, the sixth coordination site reserved for the oxygen is blocked by another histidine residue.[5]
When deoxyhemoglobin picks up an oxygen molecule, this histidine residue moves away and returns once the oxygen is securely attached to form a hydrogen bond with it. This results in the Fe2+ ion switching to a low-spin configuration, resulting in a 20% decrease in ionic radius so that now it can fit into the porphyrin ring, which becomes planar.[5] (Additionally, this hydrogen bonding results in the tilting of the oxygen molecule, resulting in a Fe–O–O bond angle of around 120° that avoids the formation of Fe–O–Fe or Fe–O2–Fe bridges that would lead to electron transfer, the oxidation of Fe2+ to Fe3+, and the destruction of hemoglobin.) This results in a movement of all the protein chains that leads to the other subunits of hemoglobin changing shape to a form with larger oxygen affinity. Thus, when deoxyhemoglobin takes up oxygen, its affinity for more oxygen increases, and vice versa.[5] Myoglobin, on the other hand, contains only one heme group and hence this cooperative effect cannot occur. Thus, while hemoglobin is almost saturated with oxygen in the high partial pressures of oxygen found in the lungs, its affinity for oxygen is much lower than that of myoglobin, which oxygenates even at low partial pressures of oxygen found in muscle tissue.[5] As described by the Bohr effect (named after Christian Bohr, the father of Niels Bohr), the oxygen affinity of hemoglobin diminishes in the presence of carbon dioxide.[5]

Carbon monoxide and phosphorus trifluoride are poisonous to humans because they bind to hemoglobin similarly to oxygen, but with much more strength, so that oxygen can no longer be transported throughout the body. Hemoglobin bound to carbon monoxide is known as carboxyhemoglobin. This effect also plays a minor role in the toxicity of cyanide, but there the major effect is by far its interference with the proper functioning of the electron transport protein cytochrome a.[5] The cytochrome proteins also involve heme groups and are involved in the metabolic oxidation of glucose by oxygen. The sixth coordination site is then occupied by either another imidazole nitrogen or a methionine sulfur, so that these proteins are largely inert to oxygen – with the exception of cytochrome a, which bonds directly to oxygen and thus is very easily poisoned by cyanide.[5] Here, the electron transfer takes place as the iron remains in low spin but changes between the +2 and +3 oxidation states. Since the reduction potential of each step is slightly greater than the previous one, the energy is released step-by-step and can thus be stored in adenosine triphosphate. Cytochrome a is slightly distinct, as it occurs at the mitochondrial membrane, binds directly to oxygen, and transports protons as well as electrons, as follows:[5]
- 4 Cytc2+ + O2 + 8H+inside → 4 Cytc3+ + 2 H2O + 4H+outside
Although the heme proteins are the most important class of iron-containing proteins, the iron–sulfur proteins are also very important, being involved in electron transfer, which is possible since iron can exist stably in either the +2 or +3 oxidation states. These have one, two, four, or eight iron atoms that are each approximately tetrahedrally coordinated to four sulfur atoms; because of this tetrahedral coordination, they always have high-spin iron. The simplest of such compounds is rubredoxin, which has only one iron atom coordinated to four sulfur atoms from cysteine residues in the surrounding peptide chains. Another important class of iron–sulfur proteins is the ferredoxins, which have multiple iron atoms. Transferrin does not belong to either of these classes.[5]
The ability of sea mussels to maintain their grip on rocks in the ocean is facilitated by their use of organometallic iron-based bonds in their protein-rich cuticles. Based on synthetic replicas, the presence of iron in these structures increased elastic modulus 770 times, tensile strength 58 times, and toughness 92 times. The amount of stress required to permanently damage them increased 76 times.[23]
Vertebrate Metabolism
In vertebrates, iron is an essential component of Hemoglobin the Oxygen transport protein.[4]
Human body iron stores

Most well-nourished people in industrialized countries have 4 to 5 grams of iron in their bodies (~38 mg iron/kg body weight for women and ~50 mg iron/kg body for men).[24] Of this, about 2.5 g is contained in the hemoglobin needed to carry oxygen through the blood (around 0.5 mg of iron per mL of blood),[25] and most of the rest (approximately 2 grams in adult men, and somewhat less in women of childbearing age) is contained in ferritin complexes that are present in all cells, but most common in bone marrow, liver, and spleen. The liver stores of ferritin are the primary physiologic source of reserve iron in the body. The reserves of iron in industrialized countries tend to be lower in children and women of child-bearing age than in men and in the elderly. Women who must use their stores to compensate for iron lost through menstruation, pregnancy or lactation have lower non-hemoglobin body stores, which may consist of 500 mg, or even less.
Of the body's total iron content, about 400 mg is devoted to cellular proteins that use iron for important cellular processes like storing oxygen (myoglobin) or performing energy-producing redox reactions (cytochromes). A relatively small amount (3–4 mg) circulates through the plasma, bound to transferrin.[26] Because of its toxicity, free soluble iron is kept in low concentration in the body.
Iron deficiency first affects the storage of iron in the body, and depletion of these stores is thought to be relatively asymptomatic, although some vague and non-specific symptoms have been associated with it. Since iron is primarily required for hemoglobin, iron deficiency anemia is the primary clinical manifestation of iron deficiency. Iron-deficient people will suffer or die from organ damage well before their cells run out of the iron needed for intracellular processes like electron transport.
Macrophages of the reticuloendothelial system store iron as part of the process of breaking down and processing hemoglobin from engulfed red blood cells. Iron is also stored as a pigment called hemosiderin, which is an ill-defined deposit of protein and iron, created by macrophages where excess iron is present, either locally or systemically, e.g., among people with iron overload due to frequent blood cell destruction and the necessary transfusions their condition calls for. If systemic iron overload is corrected, over time the hemosiderin is slowly resorbed by the macrophages.
Mechanisms of iron regulation
Human iron homeostasis is regulated at two different levels. Systemic iron levels are balanced by the controlled absorption of dietary iron by enterocytes, the cells that line the interior of the intestines, and the uncontrolled loss of iron from epithelial sloughing, sweat, injuries and blood loss. In addition, systemic iron is continuously recycled. Cellular iron levels are controlled differently by different cell types due to the expression of particular iron regulatory and transport proteins.
Systemic iron regulation
Dietary iron uptake
The absorption of dietary iron is a variable and dynamic process. The amount of iron absorbed compared to the amount ingested is typically low, but may range from 5% to as much as 35% depending on circumstances and type of iron. The efficiency with which iron is absorbed varies depending on the source. Generally, the best-absorbed forms of iron come from animal products. Absorption of dietary iron in iron salt form (as in most supplements) varies somewhat according to the body's need for iron, and is usually between 10% and 20% of iron intake. Absorption of iron from animal products, and some plant products, is in the form of heme iron, and is more efficient, allowing absorption of from 15% to 35% of intake. Heme iron in animals is from blood and heme-containing proteins in meat and mitochondria, whereas in plants, heme iron is present in mitochondria in all cells that use oxygen for respiration.
Like most mineral nutrients, the majority of the iron absorbed from digested food or supplements is absorbed in the duodenum by enterocytes of the duodenal lining. These cells have special molecules that allow them to move iron into the body. To be absorbed, dietary iron can be absorbed as part of a protein such as heme protein or iron must be in its ferrous Fe2+ form. A ferric reductase enzyme on the enterocytes' brush border, duodenal cytochrome B (Dcytb), reduces ferric Fe3+ to Fe2+.[27] A protein called divalent metal transporter 1 (DMT1), which can transport several divalent metals across the plasma membrane, then transports iron across the enterocyte's cell membrane into the cell. If the iron is bound to heme it is instead transported across the apical membrane by heme carrier protein 1 (HCP1).[28]
These intestinal lining cells can then either store the iron as ferritin, which is accomplished by Fe2+ binding to apoferritin (in which case the iron will leave the body when the cell dies and is sloughed off into feces), or the cell can release it into the body via the only known iron exporter in mammals, ferroportin. Hephaestin, a ferroxidase that can oxidize Fe2+ to Fe3+ and is found mainly in the small intestine, helps ferroportin transfer iron across the basolateral end of the intestine cells. In contrast, ferroportin is post-translationally repressed by hepcidin, a 25-amino acid peptide hormone. The body regulates iron levels by regulating each of these steps. For instance, enterocytes synthesize more Dcytb, DMT1 and ferroportin in response to iron deficiency anemia.[29] Iron absorption from diet is enhanced in the presence of vitamin C and diminished by excess calcium, zinc, or manganese.[30]
The human body's rate of iron absorption appears to respond to a variety of interdependent factors, including total iron stores, the extent to which the bone marrow is producing new red blood cells, the concentration of hemoglobin in the blood, and the oxygen content of the blood. The body also absorbs less iron during times of inflammation, in order to deprive bacteria of iron. Recent discoveries demonstrate that hepcidin regulation of ferroportin is responsible for the syndrome of anemia of chronic disease.
Iron recycling and loss
Most of the iron in the body is hoarded and recycled by the reticuloendothelial system, which breaks down aged red blood cells. In contrast to iron uptake and recycling, there is no physiologic regulatory mechanism for excreting iron. People lose a small but steady amount by gastrointestinal blood loss, sweating and by shedding cells of the skin and the mucosal lining of the gastrointestinal tract. The total amount of loss for healthy people in the developed world amounts to an estimated average of 1 mg a day for men, and 1.5–2 mg a day for women with regular menstrual periods.[31] People with gastrointestinal parasitic infections, more commonly found in developing countries, often lose more.[32] Those who cannot regulate absorption well enough get disorders of iron overload. In these diseases, the toxicity of iron starts overwhelming the body's ability to bind and store it.[33]
Cellular iron regulation
Iron import
Most cell types take up iron primarily through receptor-mediated endocytosis via transferrin receptor 1 (TFR1), transferrin receptor 2 (TFR2) and GAPDH. TFR1 has a 30-fold higher affinity for transferrin-bound iron than TFR2 and thus is the main player in this process.[34][35] The higher order multifunctional glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH) also acts as a transferrin receptor.[36][37] Transferrin-bound ferric iron is recognized by these transferrin receptors, triggering a conformational change that causes endocytosis. Iron then enters the cytoplasm from the endosome via importer DMT1 after being reduced to its ferrous state by a STEAP family reductase.[38]
Alternatively, iron can enter the cell directly via plasma membrane divalent cation importers such as DMT1 and ZIP14 (Zrt-Irt-like protein 14).[39] Again, iron enters the cytoplasm in the ferrous state after being reduced in the extracellular space by a reductase such as STEAP2, STEAP3 (in red blood cells), Dcytb (in enterocytes) and SDR2.[38]
The labile iron pool
In the cytoplasm, ferrous iron is found in a soluble, chelatable state which constitutes the labile iron pool (~0.001 mM).[40] In this pool, iron is thought to be bound to low-mass compounds such as peptides, carboxylates and phosphates, although some might be in a free, hydrated form (aqua ions).[40] Alternatively, iron ions might be bound to specialized proteins known as metallochaperones.[41] Specifically, poly-r(C)-binding proteins PCBP1 and PCBP2 appear to mediate transfer of free iron to ferritin (for storage) and non-heme iron enzymes (for use in catalysis).[39][42] The labile iron pool is potentially toxic due to iron's ability to generate reactive oxygen species. Iron from this pool can be taken up by mitochondria via mitoferrin to synthesize Fe-S clusters and heme groups.[38]
The storage iron pool
Iron can be stored in ferritin as ferric iron due to the ferroxidase activity of the ferritin heavy chain.[43] Dysfunctional ferritin may accumulate as hemosiderin, which can be problematic in cases of iron overload.[44] The ferritin storage iron pool is much larger than the labile iron pool, ranging in concentration from 0.7 mM to 3.6 mM.[40]
Iron export
Iron export occurs in a variety of cell types, including neurons, red blood cells, macrophages and enterocytes. The latter two are especially important since systemic iron levels depend upon them. There is only one known iron exporter, ferroportin.[45] It transports ferrous iron out of the cell, generally aided by ceruloplasmin and/or hephaestin (mostly in enterocytes), which oxidize iron to its ferric state so it can bind ferritin in the extracellular medium.[38] Hepcidin causes the internalization of ferroportin, decreasing iron export. Besides, hepcidin seems to downregulate both TFR1 and DMT1 through an unknown mechanism.[46] Another player assisting ferroportin in effecting cellular iron export is GAPDH.[47] A specific post translationally modified isoform of GAPDH is recruited to the surface of iron loaded cells where it recruits apo-transferrin in close proximity to ferroportin so as to rapidly chelate the iron extruded.[48]
The expression of hepcidin, which only occurs in certain cell types such as hepatocytes, is tightly controlled at the transcriptional level and it represents the link between cellular and systemic iron homeostasis due to hepcidin's role as "gatekeeper" of iron release from enterocytes into the rest of the body.[38] Erythroblasts produce erythroferrone, a hormone which inhibits hepcidin and so increases the availability of iron needed for hemoglobin synthesis.[49]
Translational control of cellular iron
Although some control exists at the transcriptional level, the regulation of cellular iron levels is ultimately controlled at the translational level by iron-responsive element-binding proteins IRP1 and especially IRP2.[50] When iron levels are low, these proteins are able to bind to iron-responsive elements (IREs). IREs are stem loop structures in the untranslated regions (UTRs) of mRNA.[38]
Both ferritin and ferroportin contain an IRE in their 5' UTRs, so that under iron deficiency their translation is repressed by IRP2, preventing the unnecessary synthesis of storage protein and the detrimental export of iron. In contrast, TFR1 and some DMT1 variants contain 3' UTR IREs, which bind IRP2 under iron deficiency, stabilizing the mRNA, which guarantees the synthesis of iron importers.[38]
Marine systems
Iron plays an essential role in marine systems and can act as a limiting nutrient for planktonic activity.[51] Because of this, too much of a decrease in iron may lead to a decrease in growth rates in phytoplanktonic organisms such as diatoms.[52] Iron can also be oxidized by marine microbes under conditions that are high in iron and low in oxygen.[53]
Iron can enter marine systems through adjoining rivers and directly from the atmosphere. Once iron enters the ocean, it can be distributed throughout the water column through ocean mixing and through recycling on the cellular level.[54] In the arctic, sea ice plays a major role in the store and distribution of iron in the ocean, depleting oceanic iron as it freezes in the winter and releasing it back into the water when thawing occurs in the summer.[55] The iron cycle can fluctuate the forms of iron from aqueous to particle forms altering the availability of iron to primary producers.[56] Increased light and warmth increases the amount of iron that is in forms that are usable by primary producers.[57]
See also
- Template:Anl known to incorporate iron into its exoskeleton
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 "Iron". Micronutrient Information Center, Linus Pauling Institute, Oregon State University, Corvallis, Oregon. April 2016. https://lpi.oregonstate.edu/mic/minerals/iron.
- ↑ 2.0 2.1 Dlouhy, Adrienne C.; Outten, Caryn E. (2013). Banci, Lucia. ed. Metallomics and the Cell. Metal Ions in Life Sciences. 12. Springer. pp. 241–78. doi:10.1007/978-94-007-5561-1_8. ISBN 978-94-007-5560-4. electronic-book ISBN:978-94-007-5561-1
- ↑ 3.0 3.1 Yee, Gereon M.; Tolman, William B. (2015). Peter M.H. Kroneck. ed. Sustaining Life on Planet Earth: Metalloenzymes Mastering Dioxygen and Other Chewy Gases. Metal Ions in Life Sciences. 15. Springer. pp. 131–204. doi:10.1007/978-3-319-12415-5_5.
- ↑ 4.0 4.1 Maton, Anthea; Jean Hopkins; Charles William McLaughlin; Susan Johnson; Maryanna Quon Warner; David LaHart; Jill D. Wright (1993). Human Biology and Health. Englewood Cliffs, New Jersey, US: Prentice Hall. ISBN 978-0-13-981176-0. https://archive.org/details/humanbiologyheal00scho.
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 5.12 5.13 5.14 5.15 Greenwood and Earnshaw, pp. 1098–104
- ↑ Wächtershäuser, Günter (1988-12-01). "Before enzymes and templates: theory of surface metabolism". Microbiol. Mol. Biol. Rev. 52 (4): 452–84. doi:10.1128/MMBR.52.4.452-484.1988. PMID 3070320.
- ↑ Wächtershäuser, G (January 1990). "Evolution of the first metabolic cycles". Proceedings of the National Academy of Sciences of the United States of America 87 (1): 200–04. doi:10.1073/pnas.87.1.200. PMID 2296579. Bibcode: 1990PNAS...87..200W.
- ↑ Günter Wächtershäuser, G (1992). "Groundworks for an evolutionary biochemistry: The iron-sulphur world". Progress in Biophysics and Molecular Biology 58 (2): 85–201. doi:10.1016/0079-6107(92)90022-X. PMID 1509092.
- ↑ Günter Wächtershäuser, G (2006). "From volcanic origins of chemoautotrophic life to Bacteria, Archaea and Eukarya". Philosophical Transactions of the Royal Society B: Biological Sciences 361 (1474): 1787–806; discussion 1806–8. doi:10.1098/rstb.2006.1904. PMID 17008219.
- ↑ Wächtershäuser, Günter (2007). "On the Chemistry and Evolution of the Pioneer Organism". Chemistry & Biodiversity 4 (4): 584–602. doi:10.1002/cbdv.200790052. PMID 17443873.
- ↑ Lippard, S.J.; Berg, J.M. (1994). Principles of Bioinorganic Chemistry. Mill Valley: University Science Books. ISBN 0-935702-73-3.
- ↑ Kikuchi, G.; Yoshida, T.; Noguchi, M. (2005). "Heme oxygenase and heme degradation". Biochemical and Biophysical Research Communications 338 (1): 558–67. doi:10.1016/j.bbrc.2005.08.020. PMID 16115609.
- ↑ "Contents of Volumes in the Metal Ions in Life Sciences Series". Metals, Microbes, and Minerals - the Biogeochemical Side of Life. De Gruyter. 2021. pp. xxv-xlvi. doi:10.1515/9783110589771-006. ISBN 9783110589771. https://www.degruyter.com/document/doi/10.1515/9783110589771-006.
- ↑ Neilands, J.B. (1995). "Siderophores: structure and function of microbial iron transport compounds". The Journal of Biological Chemistry 270 (45): 26723–26. doi:10.1074/jbc.270.45.26723. PMID 7592901.
- ↑ Neilands, J.B. (1981). "Microbial Iron Compounds". Annual Review of Biochemistry 50 (1): 715–31. doi:10.1146/annurev.bi.50.070181.003435. PMID 6455965.
- ↑ Boukhalfa, Hakim; Crumbliss, Alvin L. (2002). "Chemical aspects of siderophore mediated iron transport". BioMetals 15 (4): 325–39. doi:10.1023/A:1020218608266. PMID 12405526.
- ↑ Nanami, M.; Ookawara, T.; Otaki, Y.; Ito, K.; Moriguchi, R.; Miyagawa, K.; Hasuike, Y.; Izumi, M. et al. (2005). "Tumor necrosis factor-α-induced iron sequestration and oxidative stress in human endothelial cells". Arteriosclerosis, Thrombosis, and Vascular Biology 25 (12): 2495–501. doi:10.1161/01.ATV.0000190610.63878.20. PMID 16224057.
- ↑ Rouault, Tracey A. (2003). "How Mammals Acquire and Distribute Iron Needed for Oxygen-Based Metabolism". PLOS Biology 1 (3): e9. doi:10.1371/journal.pbio.0000079. PMID 14691550.
- ↑ "Proposed Mechanism of Catalase". Catalase: H2O2: H2O2 Oxidoreductase: Catalase Structural Tutorial. https://biology.kenyon.edu/BMB/Chime/catalase/frames/cattx.htm#Proposed%20Mechanism%20of%20Catalase.
- ↑ "The three-dimensional structure of an arachidonic acid 15-lipoxygenase". Science 260 (5113): 1482–86. 1993. doi:10.1126/science.8502991. PMID 8502991. Bibcode: 1993Sci...260.1482B.
- ↑ Gray, N.K.; Hentze, M.W. (August 1994). "Iron regulatory protein prevents binding of the 43S translation pre-initiation complex to ferritin and eALAS mRNAs". EMBO J. 13 (16): 3882–91. doi:10.1002/j.1460-2075.1994.tb06699.x. PMID 8070415.
- ↑ Gregory B. Vásquez; Xinhua Ji; Clara Fronticelli; Gary L. Gilliland (1998). "Human Carboxyhemoglobin at 2.2 Å Resolution: Structure and Solvent Comparisons of R-State, R2-State and T-State Hemoglobins". Acta Crystallogr. D 54 (3): 355–66. doi:10.1107/S0907444997012250. PMID 9761903.
- ↑ Sanderson, K (2017). "Mussels' iron grip inspires strong and stretchy polymer". Chemical & Engineering News (American Chemical Society) 95 (44): 8. doi:10.1021/cen-09544-notw3. https://cen.acs.org/articles/95/i44/Mussels-iron-grip-inspires-strong-stretchy-polymer.html. Retrieved 2 November 2017.
- ↑ Gropper, Sareen S.; Smith, Jack L. (2013). Advanced Nutrition and Human Metabolism (6th ed.). Belmont, CA: Wadsworth. p. 481. ISBN 978-1133104056. https://books.google.com/books?id=3R0Yeu79jfQC&pg=PA481.
- ↑ Truswell, A. Stewart (2010-07-15) (in en). ABC of Nutrition. John Wiley & Sons. pp. 52. ISBN 9781444314229. https://books.google.com/books?id=01JfkuEaL4EC&pg=PA52.
- ↑ "Regulation of iron balance". UpToDate. 2011-11-07. http://www.uptodate.com/contents/regulation-of-iron-balance.
- ↑ "An iron-regulated ferric reductase associated with the absorption of dietary iron". Science 291 (5509): 1755–9. Mar 2001. doi:10.1126/science.1057206. PMID 11230685. Bibcode: 2001Sci...291.1755M.
- ↑ Rouault, Tracey A. (2005-09-09). "The Intestinal Heme Transporter Revealed" (in en). Cell 122 (5): 649–651. doi:10.1016/j.cell.2005.08.027. ISSN 0092-8674. PMID 16143096.
- ↑ "Orchestration of iron homeostasis". The New England Journal of Medicine 352 (17): 1741–4. Apr 2005. doi:10.1056/NEJMp048363. PMID 15858181.
- ↑ Abbaspour, Nazanin (Feb 2014). "Review on iron and its importance for human health". J Res Med Sci 19 (2): 164–174. PMID 24778671.
- ↑ Janet, R Hunt (June 2009). "Body iron excretion by healthy men and women". The American Journal of Clinical Nutrition 89 (6): 1792–1798. doi:10.3945/ajcn.2009.27439. PMID 19386738.
- ↑ "Disorders of iron metabolism". The New England Journal of Medicine 342 (17): 1293–4. Apr 2000. doi:10.1056/NEJM200004273421716. PMID 10787338.
- ↑ "Iron overload syndromes other than hereditary hemochromatosis". UpToDate. 2011-11-07. http://www.uptodate.com/contents/iron-overload-syndromes-other-than-hereditary-hemochromatosis.
- ↑ "Transferrin receptor 2-alpha supports cell growth both in iron-chelated cultured cells and in vivo". The Journal of Biological Chemistry 275 (22): 16618–25. June 2000. doi:10.1074/jbc.M908846199. PMID 10748106.
- ↑ "Comparison of the interactions of transferrin receptor and transferrin receptor 2 with transferrin and the hereditary hemochromatosis protein HFE". The Journal of Biological Chemistry 275 (49): 38135–8. December 2000. doi:10.1074/jbc.C000664200. PMID 11027676.
- ↑ "Characterization of glyceraldehyde-3-phosphate dehydrogenase as a novel transferrin receptor". The International Journal of Biochemistry & Cell Biology 44 (1): 189–99. Jan 2012. doi:10.1016/j.biocel.2011.10.016. PMID 22062951.
- ↑ "Secreted glyceraldehye-3-phosphate [sic] dehydrogenase is a multifunctional autocrine transferrin receptor for cellular iron acquisition". Biochimica et Biophysica Acta (BBA) - General Subjects 1830 (6): 3816–27. Jun 2013. doi:10.1016/j.bbagen.2013.03.019. PMID 23541988.
- ↑ 38.0 38.1 38.2 38.3 38.4 38.5 38.6 "Two to tango: regulation of Mammalian iron metabolism". Cell 142 (1): 24–38. Jul 2010. doi:10.1016/j.cell.2010.06.028. PMID 20603012.
- ↑ 39.0 39.1 Lane, D.J.R.; Merlot, A.M.; Huang, M.L.-H.; Bae, D.-H.; Jansson, P.J.; Sahni, S.; Kalinowski, D.S.; Richardson, D.R. (May 2015). "Cellular iron uptake, trafficking and metabolism: Key molecules and mechanisms and their roles in disease". Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 1853 (5): 1130–1144. doi:10.1016/j.bbamcr.2015.01.021. PMID 25661197.
- ↑ 40.0 40.1 40.2 Yehuda, Shlomo; Mostofsky, David I., eds (2010). Iron Deficiency and Overload From Basic Biology to Clinical Medicine. Nutrition and Health. New York, NY: Humana Press. p. 230. doi:10.1007/978-1-59745-462-9. ISBN 9781934115220. https://www.springer.com/gp/book/9781934115220.
- ↑ "Transition metal speciation in the cell: insights from the chemistry of metal ion receptors". Science 300 (5621): 931–6. May 2003. doi:10.1126/science.1085049. PMID 12738850. Bibcode: 2003Sci...300..931F.
- ↑ Philpott, Caroline C.; Ryu, Moon-Suhn (22 July 2014). "Special delivery: distributing iron in the cytosol of mammalian cells". Frontiers in Pharmacology 5: 173. doi:10.3389/fphar.2014.00173. PMID 25101000.
- ↑ "Ferritin, iron homeostasis, and oxidative damage". Free Radical Biology & Medicine 33 (4): 457–63. Aug 2002. doi:10.1016/s0891-5849(02)00842-0. PMID 12160928.
- ↑ "Brain iron metabolism". Seminars in Pediatric Neurology 13 (3): 142–8. Sep 2006. doi:10.1016/j.spen.2006.08.002. PMID 17101452.
- ↑ "Cellular iron: ferroportin is the only way out". Cell Metabolism 1 (3): 155–7. Mar 2005. doi:10.1016/j.cmet.2005.02.005. PMID 16054057.
- ↑ "Hepcidin directly inhibits transferrin receptor 1 expression in astrocytes via a cyclic AMP-protein kinase A pathway". Glia 59 (6): 936–45. Jun 2011. doi:10.1002/glia.21166. PMID 21438013.
- ↑ Boradia, Vishant Mahendra; Raje, Manoj; Raje, Chaaya Iyengar (1 December 2014). "Protein moonlighting in iron metabolism: glyceraldehyde-3-phosphate dehydrogenase (GAPDH)". Biochemical Society Transactions 42 (6): 1796–1801. doi:10.1042/BST20140220. PMID 25399609.
- ↑ "Moonlighting cell-surface GAPDH recruits apotransferrin to effect iron egress from mammalian cells". Journal of Cell Science 127 (Pt 19): 4279–91. Oct 2014. doi:10.1242/jcs.154005. PMID 25074810.
- ↑ "Identification of erythroferrone as an erythroid regulator of iron metabolism". Nature Genetics 46 (7): 678–84. Jul 2014. doi:10.1038/ng.2996. PMID 24880340.
- ↑ "Systemic iron homeostasis and the iron-responsive element/iron-regulatory protein (IRE/IRP) regulatory network". Annual Review of Nutrition 28: 197–213. 2008. doi:10.1146/annurev.nutr.28.061807.155521. PMID 18489257.
- ↑ Morel, François M. M.; Hudson, Robert J. M.; Price, Neil M. (1991). "Limitation of productivity by trace metals in the sea". Limnology and Oceanography 36 (8): 1742–1755. doi:10.4319/lo.1991.36.8.1742. Bibcode: 1991LimOc..36.1742M.
- ↑ Brzezinski, Mark A.; Baines, Stephen B.; Balch, William M.; Beucher, Charlotte P.; Chai, Fei; Dugdale, Richard C.; Krause, Jeffrey W.; Landry, Michael R. et al. (2011). "Co-limitation of diatoms by iron and silicic acid in the equatorial Pacific". Deep Sea Research Part II: Topical Studies in Oceanography 58 (3–4): 493–511. doi:10.1016/j.dsr2.2010.08.005. Bibcode: 2011DSRII..58..493B.
- ↑ Field, E. K.; Kato, S.; Findlay, A. J.; MacDonald, D. J.; Chiu, B. K.; Luther, G. W.; Chan, C. S. (2016). "Planktonic marine iron oxidizers drive iron mineralization under low-oxygen conditions". Geobiology 14 (5): 499–508. doi:10.1111/gbi.12189. PMID 27384464.
- ↑ Wells, Mark L.; Price, Neil M.; Bruland, Kenneth W. (1995). "Iron chemistry in seawater and its relationship to phytoplankton: A workshop report". Marine Chemistry 48 (2): 157–182. doi:10.1016/0304-4203(94)00055-I.
- ↑ Lannuzel, D.; Vancoppenolle, M.; Van Der Merwe, P.; De Jong, J.; Meiners, K.M.; Grotti, M.; Nishioka, J.; Schoemann, V. (2016). "Iron in sea ice: Review and new insights". Elementa: Science of the Anthropocene 4. doi:10.12952/journal.elementa.000130.
- ↑ Raiswell, R. (2011). "Iron Transport from the Continents to the Open Ocean: The Aging-Rejuvenation Cycle". Elements 7 (2): 101–106. doi:10.2113/gselements.7.2.101.
- ↑ Tagliabue, Alessandro; Bopp, Laurent; Aumont, Olivier; Arrigo, Kevin R. (2009). "Influence of light and temperature on the marine iron cycle: From theoretical to global modeling". Global Biogeochemical Cycles 23 (2). doi:10.1029/2008GB003214. Bibcode: 2009GBioC..23.2017T. https://hal.archives-ouvertes.fr/hal-00413634/file/2008GB003214.pdf.
 |